CBE Faculty Take Lead on Two Major Research Initiatives
CBE Professors Fanxing Li, Henry Lamb and Phil Westmoreland have received funding for research work that centers on using newly-developed oxide materials that significantly improve the efficiency of two industrially-important chemical process operations. The funding is in the form of two major research grants.
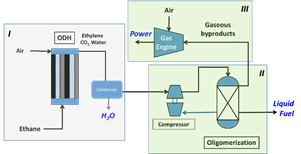
The first grant, approximately $4 million over three years, including cost share ($1.36 million from DOE), involves all three faculty members (Professor Li is the principal investigator) and is focused on manufacturing high-value gasoline from ethane that’s currently a waste by-product of shale gas production.
The work is funded by the Rapid Advancement in Process Intensification Deployment Institute (RAPID). RAPID is one of several research institutes created by the federal government with the mission of increasing U.S. manufacturing competitiveness. Manufacturing USA is the umbrella organization for the institutes.
Natural gas containing more than 10 volume percent ethane can’t be shipped using interstate pipelines, and shale gas can contain ethane levels as high as 20 volume percent. That leaves small scale shale gas operators in the lower 48 states needing to dispose of more than 210 million barrels (liquid equivalent) of ethane that are rejected each year. Typically, the excess ethane is flared, which adds to the global burden of atmospheric CO2.
The researchers’ approach to solving the glut is upgrading the low-to-negative-value ethane and other natural gas liquids (NGLs) to an easily transportable high-value liquid fuel, synthetic gasoline. Since shale gas operations are typically small and in isolated locations, the key to the process economics is development of modular systems that can operate economically at stranded sites.
With that in mind, the project team aims to develop small-scale units that produce gasoline with 90% capital cost savings and up to 80% reduction in energy demand (for ethane conversion), relative to existing gasoline production operations. The use of advanced reaction and reactive-separation methods that rely on new oxide catalysts synthesized by the Li research group will simplify the process scheme and reduce energy requirements.
The research team is partnering with EcoCatalytic Technologies LLC on the project.
The second grant was awarded to Professor Li for $2.05 million over three years by the Department of Energy’s National Energy Technology Laboratory.
“This project is aimed at developing a less expensive, more efficient means of extracting oxygen from the air – which is important given oxygen’s utility for chemical manufacturing and energy production applications,” says Li, the PI of the grant.
At present, oxygen extraction usually involves cooling air to extremely low temperatures – around -185 degrees Celsius. Li and his research team plan to use oxygen-sorbent material tailored to remove oxygen from heated air, with the idea that the material would then release the captured oxygen into an environment at lower oxygen partial pressures.
“This would eliminate nitrogen from the air, creating an oxygen-rich product that could be easily refined based on the needs of the end user,” Li says.
The research will be carried out by a project team with extensive and complementing expertise in the areas of oxygen sorbent development and testing (Li research group), mixed oxide characterization and modeling (Professor Xingbo Liu of West Virginia University), and redox based air separation process development and demonstrations (Dr. Vijay Sethi of Thermosolv LLC).
The original article was written by Matt Shipman, Research Lead in University Communications at NC State.