Professor Abolhasani and Colleagues Develop Platform for High-Throughput Reaction Screening
Members of Professor Milad Abolhasani’s research group and their off-campus colleagues have developed a microscale flow-based, high-throughput screening technology that offers a faster, safer and less expensive means of identifying optimum conditions for performing high-pressure/high-temperature catalytic chemical reactions. The technique focuses on hydroformylation reactions, which are used to create a variety of commercial products.
“Hydroformylation reactions are industrial processes that are used to make everything from plasticizers to detergents,” says Professor Abolhasani, corresponding author of a paper on the work. “The testing and analysis process for evaluating a single set of conditions using conventional techniques normally takes days. We can now do it in about 30 minutes.”
“Eastman uses the homogenous hydroformylation process to make secondary materials included in many products that enhance our quality of life in a material way, such as paints, pharmaceuticals and inks,” says Dawn Mason, the external innovation manager at Eastman Chemical Company. “Being able to provide technological improvements in a safer and more expeditious manner than previously available is what makes our partnership with NC State successful.”
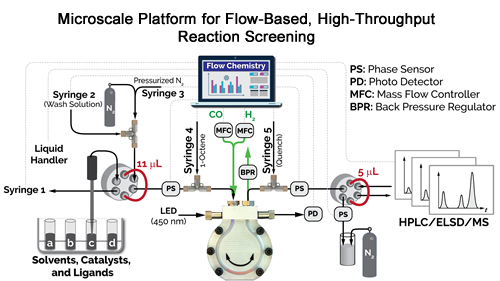 The new technique uses extremely small samples – on the order of 11 microliters, rather than the milliliters used in conventional techniques. The technique also integrates reagent preparation, reaction processes and analysis into a single sequence. An explanatory video regarding the work is available here.
The new technique uses extremely small samples – on the order of 11 microliters, rather than the milliliters used in conventional techniques. The technique also integrates reagent preparation, reaction processes and analysis into a single sequence. An explanatory video regarding the work is available here.
“Most optimization processes involve multiple steps that are conducted at different work stations,” Abolhasani says. “That’s one reason they take so long. By integrating these steps into a single, continuous sequence, we’ve made the process significantly more efficient.”
The high-throughput flow chemistry technique and the smaller sample size also expedite the speed of the reaction. But speed isn’t the only advantage – the new technique is also safer. That’s because these reactions have to be conducted under high pressures, and at high temperatures, using toxic and flammable gases.
“Our technique minimizes human interaction with these gases, since most of the work is done by robots,” Abolhasani says. “Also, we use only 60 microliters of these gases, where conventional techniques use a few milliliters or more – that’s a difference of at least two orders of magnitude, and that means our process is safer.”
The relatively small sample sizes also save money. The catalysts and ligands used in the reactions are expensive. By using smaller samples, the process requires less amount of expensive ligands and catalyst material, reducing the relevant expense by two to three orders of magnitude.
“Ultimately, developing a more efficient technique for these reactions is important because it expedites R&D, allowing researchers to both improve manufacturing processes and to accelerate industry’s ability to identify new ligands that have commercial applications,” Abolhasani says.
The paper, “Flow Chemistry-Enabled Studies of Rhodium-Catalyzed Hydroformylation Reactions,” is published in the Royal Society of Chemistry journal Chemical Communications. First author on the paper is Cheng Zhu, a CBE postdoctoral researcher. Co-authors include Keshav Raghuvanshi, a CBE postdoc; Connor Coley of MIT; and Dawn Mason, Jody Rodgers and Mesfin Janka of Eastman Chemical Company. Eastman Chemical Company funded the research.
Except for particularization to CBE, this article was written by Matt Shipman, Research Lead in University Communications at NC State.
- Categories:


