Creating a New Way to Remove Phosphorus from Contaminated Water

Professor Jan Genzer and his colleagues have created an inexpensive hydrogel that can filter phosphorus from contaminated surface waters, drinking water supplies or wastewater streams to reduce phosphorus pollution and reuse the phosphorus for agricultural and industrial applications.
A hydrogel is a three-dimensional, cross-linked network of hydrophilic polymers that can absorb and retain water or other fluids. They are known for their soft, jelly-like consistency and ability to retain water. In addition to efficiently capturing and releasing phosphorus, the new hydrogels can be reused multiple times – making them cost-effective.
Phosphorus is an essential element for many applications, particularly agricultural fertilizers. But there are two key challenges. First, the phosphorus used for farming and industrial applications primarily comes from mining operations, which rely on limited resources and can pose environmental problems. Second, high phosphorus levels in surface waters – from agricultural runoff, wastewater plants and other sources – contribute to significant water-quality problems such as eutrophication, leading to so-called “dead zones.”
“The idea of filtering phosphorus from contaminated waters is not new, but existing technologies rely on potent acids or bases to release the phosphorus once it has been captured,” says Prof. Genzer, co-corresponding author of a paper on the work, and S. Frank and Doris Culberson Distinguished Professor of Chemical and Biomolecular Engineering. “Ultimately, this poses environmental challenges of its own and makes it expensive to harvest phosphorus using filtration technologies. We have made major strides toward solving this problem.”
The researchers created a hydrogel that combines two commercially available materials: polyethyleneimine (PEI), which is an inexpensive polymer whose molecular structure allows it to harvest phosphorus from water as the water passes through the material, and poly(methyl vinyl ether-co-maleic anhydride) (PMVEMA), which is an inexpensive polymer that bonds with the PEI to form a robust gel that allows water to pass through while maintaining its structural integrity.
In testing, the PEI/PMVEMA hydrogel was extremely efficient at removing phosphorus from contaminated water as it flowed through the material at room temperature. It also efficiently released the captured phosphorus at room temperature using mild bases.
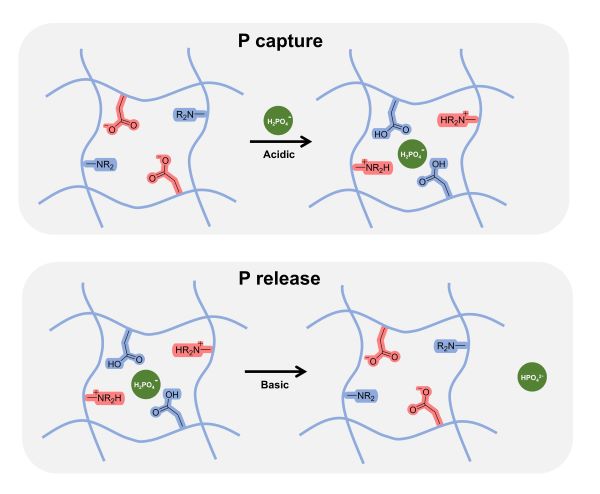
“Our experiments suggest the hydrogel would be able to remove well over 90% of the phosphorus from wastewater or contaminated surface waters,” Genzer says. “We also demonstrated that we can reclaim up to 99% of that phosphorus for reuse. We also showed that the hydrogel can then be reused with minimal decline in performance. For example, after being used three times, we could still reuse 97.5% of the phosphorus.
“To put this in context, current phosphorus filtration materials cost $20-300 per pound of phosphorus they can capture,” says Genzer. “Our material costs $23 per pound of phosphorus removed if you only use it once. But you can use it over and over again. If you use the hydrogel twice, the cost drops to $8 per pound of phosphorus harvested. If you use it 50 times, the price drops to less than 50 cents per pound.
“We have filed a provisional patent for the material and are looking for industry partners interested in incorporating the material into practical applications for wastewater treatment, environmental remediation, and harvesting phosphorus for agricultural and industrial use,” says Genzer.
“From a research standpoint, the next big challenge is determining how to use this material to harvest phosphorus from contaminated soils. That is a more complex problem than removing phosphorus from liquids.”
The paper, “Functional Hydrogels for Selective Phosphate Removal from Water and Release on Demand,” is published in Langmuir. The paper’s first author is Jiangfeng Xu, a Ph.D. student at NC State. The paper’s co-corresponding author is Kirill Efimenko, a research associate professor working in Prof. Genzer’s research group. The paper was co-authored by Christopher Gorman, a professor of chemistry at NC State; Yaroslava Yingling, Kobe Steel Distinguished Professor of Materials Science and Engineering at NC State; and Lisa Castellano, a research associate at NC State.
This work was supported by the Science and Technologies for Phosphorus Sustainability (STEPS) Center, a National Science Foundation Science and Technology Center based at NC State that is funded under grant CBET-2019435.
This article is a modified version of an article written by Matt Simpson for the NC State Office of Research and Innovation.